Bovilis® Vista Once SQ
Bovilis® Vista Once SQ contains modified live cultures of bovine rhinotracheitis (IBR) virus, bovine virus diarrhoea (BVD) virus (Type 1 and 2), parainfluenza3 virus (PI3), bovine respiratory syncytial virus (BRSV) and avirulent live cultures of Mannheimia haemolytica and Pasteurella multocida.
FOR ANIMAL USE ONLY
BOVILIS ® VISTA ONCE SQ
Reg. No. G4061 (Act 36/1947)
Cattle vaccine that contains modified live cultures of bovine rhinotracheitis (IBR) virus, bovine virus diarrhoea (BVD) virus (types 1 and 2), parainfluenza 3 virus (PI3), bovine respiratory syncytial virus (BRSV) and avirulent live cultures of Mannheimia haemolytica and Pasteurella multocida.
INDICATIONS
For the vaccination of healthy cattle, 3 months of age and older, as an aid in the prevention of respiratory disease caused by infectious bovine rhinotracheitis (IBR) virus, bovine virus diarrhoea (BVD) virus types 1 and 2 and bovine respiratory syncytial virus (BRSV); and as an aid in the control of disease caused by bovine virus diarrhoea (BVD) virus, parainfluenza3 virus (PI3), Mannheimia haemolytica and Pasteurella multocida.
Additionally, Bovilis® Vista Once SQ is for the vaccination of healthy cows and heifers prior to breeding as an aid in the prevention of foetal infection, including persistently infected calves, caused by BVD virus (types 1 and 2) and as an aid in the reduction of abortion due to IBR.
Reproductive duration of immunity (DOI) has been demonstrated to be at least 217 days for IBR and at least 206 days for BVD (types 1 and 2).
Respiratory duration of immunity has been demonstrated to be at least 365 days for IBR,
at least 365 days for BVD (type 1) and at least 365 days for BVD (type 2).
Calves nursing immune dams should be vaccinated from the age of 3 months when maternal antibody levels will allow active immunization.
COMPOSITION
| Active Ingredients | Quantity per 2 mℓ dose |
| Infectious bovine rhinotracheitis (IBR) virus | ≥10 3,6 TCID50 |
| Bovine virus diarrhoea virus type 1 (BVD1) | ≥10 3,8 TCID50 |
| Bovine virus diarrhoea virus type 2 (BVD2) | ≥10 3,5 TCID50 |
| Parainfluenza3 virus (PI3) | ≥10 5,1 TCID50 |
| Bovine respiratory syncytial virus (BRSV) | ≥10 3,8 TCID50 |
| Mannheimia (Pasteurella) haemolytica | ≥ 6 x 105 CFU |
| Pasteurella multocida | ≥ 6 x 105 CFU |
This product may contain residual streptomycin from the bacterial culture media (necessary growth factor) and residual penicillin/streptomycin from the cell culture media (preservative during virus propagation).
STORAGE
- Store between 2 °C and 7 °C.
- Do not freeze.
WARNINGS
- Withdrawal period: Do not slaughter within 21 days after vaccination.
- Contains residual penicillin and streptomycin as preservative.
- Use immediately after reconstitution, do not save unused contents.
- Do not dispose via wastewater or household waste. Destroy any unused vaccine and dispose of all the vaccine containers and disposable equipment after use in accordance with National Environmental Management: Waste Act, 2008 (Act No. 59 of 2008).
- Do not mix vaccine with other veterinary medicinal products.
- KEEP OUT OF REACH OF CHILDREN, UNINFORMED PERSONS AND ANIMALS.
- Although this vaccine has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
USE DURING PREGNANCY AND LACTATION
Safe for use in pregnant heifers and cows, or calves nursing pregnant cows, provided the cows and heifers in the herd are vaccinated prior to breeding, within the previous 12 months, with any of the modified live IBR and BVD containing vaccine(s) in this product line.
PRECAUTIONS
Special precautions for use in animals.
- Vaccinate healthy cattle only.
- Calves nursing immune dams should be vaccinated from the age of 3 months when maternal antibody levels will allow active immunisation.
- Foetal health risks associated with vaccination of pregnant animals with modified live vaccines cannot unequivocally be determined by clinical trials conducted for licensure. Management strategies based on vaccination of pregnant animals with modified live vaccines should be discussed with a veterinarian.
Special precautions to be taken by the person administering the vaccine to
animals.
- The transfer needle is sharp and may cause injury to self or animals if not handled properly or disposed of properly.
- In case of accidental self-injection seek medical advice immediately and show this package insert or label to the doctor.
DOSAGE AND DIRECTIONS FOR USE – USE ONLY AS DIRECTED
- Inject 2 mℓ of vaccine subcutaneously to healthy cattle 3 months of age or older.
- Annual revaccination is recommended.
- A revaccination dose can be administered at more frequent intervals based upon individual farm disease risk assessment or anytime epidemic conditions exist or are reported. Consult your veterinarian.
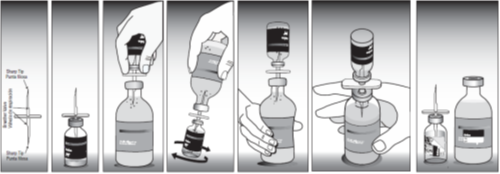
Mixing directions
5 and 10 dose presentation:
- Rehydrate freeze dried vial of Bovilis® Vista Once SQ with the enclosed diluent.
- Mix reconstituted vial well.
50 and 100 dose presentation:
- Rehydrate freeze dried vial of Bovilis® Vista Once SQ with part of the enclosed diluent using the transfer needle provided (see pictorial directions).
- Mix reconstituted vial well and transfer rehydrated vaccine into diluent vial and mix reconstituted vial well.
- Peel label from bottle of Bovilis® Vista Once SQ and place on diluent bottle containing all the vaccine.
ADVERSE REACTIONS
- If anaphylactic reaction occurs, treat with epinephrine.
- Slight transient reactions at the site of injection may occur.
- Consult your veterinarian.
PRESENTATION
Vaccine is presented in glass vials with rubber stoppers, containing 5, 10, 50 or 100 doses.
Diluent is presented in glass or plastic vials or bags, containing 10, 20, 100 or 200 mℓ.
Not all pack sizes may necessarily be marketed.
REGISTRATION HOLDER
Intervet South Africa (Pty) Ltd.
20 Spartan Road
Spartan, 1619
Tel: +27 (0) 11 923 9300
E-mail: msdahza@msd.com
www.msd-animal-health.co.za
DATE OF PUBLICATION OF THIS PACKAGE INSERT
25 April 2016
