REVALOR® XR
FOR ANIMAL USE ONLY
REVALOR® XR
Reg. No. G4490 (Act 36/1947)
INDICATIONS
Revalor® XR is indicated for increased rate of weight gain and improved feed efficiency during 70 to 200 days after implantation in steers and heifers fed in confinement for slaughter.
COMPOSITION
Each implant* contains:
200 mg trenbolone acetate
20 mg estradiol
*1 implant = 10 pellets
Excipients:
Cholesterol, ethylcellulose N200, magnesium stearate, ethanol.
STORAGE INSTRUCTIONS
- Store unopened Revalor® XR at or below 25 °C in the original packaging.
- Do not freeze.
- Avoid excessive heat and humidity.
- Use Revalor® XR before the expiry date.
- Opened cartridges may be stored in the foil pouch, in the refrigerator between 2 °C and 8 °C, protected from light, for up to 6 months.
WARNINGS
- Withdrawal period: NO withdrawal period is required when used according to the label.
- DO NOT USE IN ANIMALS INTENDED FOR SUBSEQUENT BREEDING, OR IN LACTATING DAIRY COWS.
- Do not administer implants more than once.
- Do not use in calves to be processed for veal.
- Do not administer to pre-ruminating calves, as a withdrawal period has not been established for this use.
- Handle Revalor® XR with care, as it is harmful if swallowed.
- Implant tablets subcutaneously on the back of the ear only.
- Do not use implant site for human or animal consumption.
- KEEP OUT OF REACH OF CHILDREN, UNINFORMED PERSONS AND ANIMALS.
- Although this remedy has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
PRECAUTIONS
- Store away from food and feed.
- Wear suitable protective clothing for administration of implants.
- Dispose of any containers, disposable equipment and any other waste after use in accordance with National Environmental Management Waste Act 59, 2008.
- Do not eat, drink or smoke whilst handling Revalor® XR.
- DO NOT USE IN HUMANS.
- Do not contaminate water sources with disposable materials or implants.
DIRECTIONS FOR USE – USE ONLY AS DIRECTED
Dosage and Administration
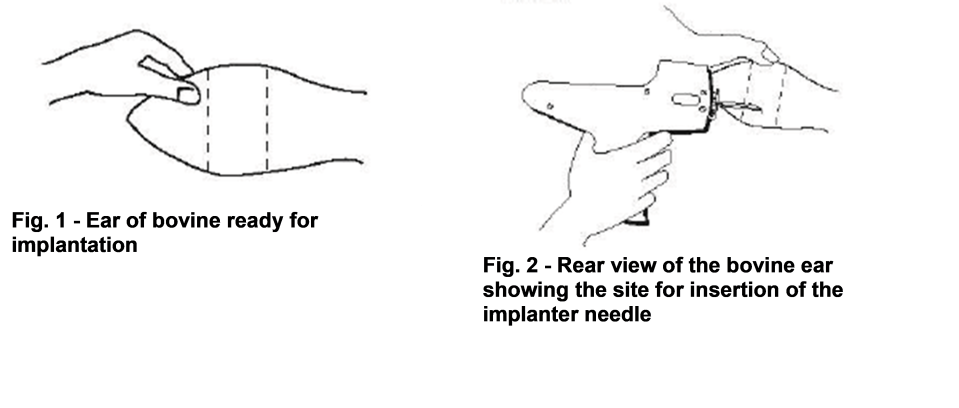
The multiple dose cartridge consists of 10 implants each containing 10 small pellets. The pellets are coated with a polymer to provide both extended and delayed release of the active ingredients. A single implant is placed under the skin, on the posterior aspect of the ear
(Fig. 1), by means of a special implanter. The implanter is available from Intervet South Africa (Pty) Ltd. (refer to implanter instructions).
Site of implantation
After appropriately restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site with a suitable sanitiser.
The site of implantation is the soft skin on the posterior aspect of the ear.
The implant should be placed as far from the head as possible without inserting the implanter into the area where the skin is firmly attached to the cartilage.
THE IMPLANT MUST NOT BE PLACED CLOSER TO THE HEAD THAN THE EDGE OF THE CARTILAGE RING FARTHEST FROM THE HEAD.
The location of insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site (Fig. 2).
Care should be taken to avoid injury to the major blood vessels or cartilage of the ear.
PRESENTATION
Each implant consists of 10 small light yellow, cylindrical pellets with beveled edges.
Revalor® XR is available in a container of 10 x 10 cartridge implants. It contains 100 doses in 10 cartridges with 10 implants per cartridge.
REGISTRATION HOLDER
Intervet South Africa (Pty) Ltd.
20 Spartan Road, Spartan
1619, RSA
Tel: +27 (0) 11 923 9300
E-mail:msdahza@msd.com
MANUFACTURER
Intervet GesmbH
Siemensstraße
107 A-1210 Vienna
Austria
DATE OF PUBLICATION OF THIS PACKAGE INSERT
01 March 2022
